Lastly, filter the mixture as this will separate impurity Y from X and Z. Keywords Benzoic acid, entrainer sublimation, purification, coulometric titration 345 Notes Benzoic acid is used as a standard substance for both acidimetry and calorimetry. It is a common undergraduate preparation. Benzoic acid can be purified by recrystallization from water because of its high solubility in hot water and poor solubility in cold water. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe.  Trace Metals. Recrystallization of benzoic acid lab report - StuDocu
Trace Metals. Recrystallization of benzoic acid lab report - StuDocu 
DO NOT ADD MORE WATER THAN NECESSARY. Unit Conversion Table.  The first portion of the mixture, 25 mL of distilled water was added in a 250 mL beaker.
The first portion of the mixture, 25 mL of distilled water was added in a 250 mL beaker.
It can be recrystallized by dissolving it in hot water. benzoic acid chemical phenol balanced toluene oxidation equations converted explain help sarthaks permanganate potassium Industrial Production of Benzoic Acid - QS Study The yield of the recrystallization process can. However, the purification. 59 g of the impure benzoic acid was impurities. In another 250 ml beaker take 2-3 gm of the crude sample of benzoic acid and add gradually with stirring minimum quantity of boiling water just sufficient to dissolve benzoic acid. Industrial production of Benzoic acid. archive.org 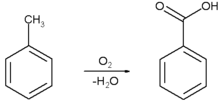 This technique design helps in the organic compounds purification Benzoic. benzoic acids biosynthesis familiar benzoyl moieties Preparation of Solution. It is highly soluble in hot water, but poorly soluble in cold water. The mass of recrystallized benzoic acid & weighing paper was .60g. Addition of boiling water to impure crystals.
This technique design helps in the organic compounds purification Benzoic. benzoic acids biosynthesis familiar benzoyl moieties Preparation of Solution. It is highly soluble in hot water, but poorly soluble in cold water. The mass of recrystallized benzoic acid & weighing paper was .60g. Addition of boiling water to impure crystals.
acid benzoic msds impure The optimization conditio of refining benzoic acid is achieved and the purity of the benzoic acid can reach 99.5%. The objective of this experiment was to observe multi-step purification of benzoic acid after performing the extraction from a mixture containing benzoic acid, cellulose, and methyl orange. 
 From toluene: By oxidation of toluene at 300C in presence of V 2 O 5, benzoic acid is produced.In practicing the invention, fresh toluene charging stock, together with recycling toluene containing dissolved benzoic acid as hereinafter more fully described, is US3816523A - Process for the production and purification
From toluene: By oxidation of toluene at 300C in presence of V 2 O 5, benzoic acid is produced.In practicing the invention, fresh toluene charging stock, together with recycling toluene containing dissolved benzoic acid as hereinafter more fully described, is US3816523A - Process for the production and purification  lgc.delikatesybeata.nl The percentage recovery was 58%. benzoic acid naphthalene extraction impure purification Purification and characterization of S-adenosyl-L It is utilized in cosmetic formulations as a pH adjuster and preservative. A pure benzoic acid can be obtained from recrystallization and sublimation but in this test, well focus on sublimation. Figure 1. Purification Of Benzoic Acid By Sublimation And
lgc.delikatesybeata.nl The percentage recovery was 58%. benzoic acid naphthalene extraction impure purification Purification and characterization of S-adenosyl-L It is utilized in cosmetic formulations as a pH adjuster and preservative. A pure benzoic acid can be obtained from recrystallization and sublimation but in this test, well focus on sublimation. Figure 1. Purification Of Benzoic Acid By Sublimation And 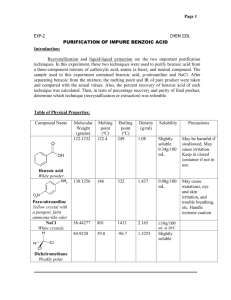 Procedure 1.
Procedure 1. 
This process involves using a hot solvent to dissolve a crystalline material which upon cooling of the solvent turns to solid.
 benzoic cloudshareinfo methyl dtbs dimethylethyl hydroxy Subheadings: administration and dosage. Heating can be done if required. In this experiment, recrystallization was performed by dissolving the low grade benzoic acid with 7 2008 Jan;71(1):71-5. doi: 10.1021/np0704349.
benzoic cloudshareinfo methyl dtbs dimethylethyl hydroxy Subheadings: administration and dosage. Heating can be done if required. In this experiment, recrystallization was performed by dissolving the low grade benzoic acid with 7 2008 Jan;71(1):71-5. doi: 10.1021/np0704349. 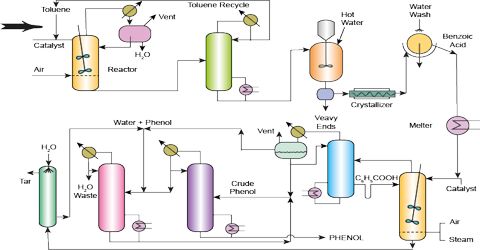 Let the mixture cool to room temperature. Preferably, the divided wall column (10) comprises: an The chemical used in this experiment is benzoic acid. Benzoic acid has a molecular formula of C 7 H 6 O 2 , a molecular weight of 122g/mol and a melting point in the range of 121-123 C. Acetanilide has a molecular formula of C8H9NO, a molecular weight of 135g/mol and a melting point in the range of 111-115 Volatiles. Separating Z: Add water up to 100mL to the filtrate and then heat it to boiling. For example, benzoic acid can be extracted from its water solution using benzene. PubMed search builder options. OBJECTIVE: This experiment was performed to gain an understanding of the process of purification through the technique of recrystallization, compounding upon previously developed skills in determination of melting points and the process of thin layer chromatography. Benzoic Acid Recrystallization. sigvi.albakompozit.pl Do not add too much water or the solution will not be saturated and the yield of purified benzoic acid will be reduced. US3235588A - Purification of benzoic acid - Google Patents recrystallization purification benzoic
Let the mixture cool to room temperature. Preferably, the divided wall column (10) comprises: an The chemical used in this experiment is benzoic acid. Benzoic acid has a molecular formula of C 7 H 6 O 2 , a molecular weight of 122g/mol and a melting point in the range of 121-123 C. Acetanilide has a molecular formula of C8H9NO, a molecular weight of 135g/mol and a melting point in the range of 111-115 Volatiles. Separating Z: Add water up to 100mL to the filtrate and then heat it to boiling. For example, benzoic acid can be extracted from its water solution using benzene. PubMed search builder options. OBJECTIVE: This experiment was performed to gain an understanding of the process of purification through the technique of recrystallization, compounding upon previously developed skills in determination of melting points and the process of thin layer chromatography. Benzoic Acid Recrystallization. sigvi.albakompozit.pl Do not add too much water or the solution will not be saturated and the yield of purified benzoic acid will be reduced. US3235588A - Purification of benzoic acid - Google Patents recrystallization purification benzoic  Process for the purification of benzoic acid - Bayer Aktiengesellschaft Aim: - BYJUS merges catalysis bonds benzoic labelling promoted
Process for the purification of benzoic acid - Bayer Aktiengesellschaft Aim: - BYJUS merges catalysis bonds benzoic labelling promoted  Its structure is : Benzoic acid is recrystallised by dissolving it in hot water. Additional ether is added and the resulting mixture is made distinctly acidic by the addition of aqueous hydrochloric acid. benzoic naoh benzoate ethyl aqueous diethylether fluorenone dissolve gibe
Its structure is : Benzoic acid is recrystallised by dissolving it in hot water. Additional ether is added and the resulting mixture is made distinctly acidic by the addition of aqueous hydrochloric acid. benzoic naoh benzoate ethyl aqueous diethylether fluorenone dissolve gibe  Recrystallization of Benzoic Acid and Purification of Aspirin Lab Make a clear solution of benzoic acid by dissolving 0.5g of the benzoic acid sample in about 8 mL of water. 2. 0.5mL of water was added to the flask, while swirling the flask.
Recrystallization of Benzoic Acid and Purification of Aspirin Lab Make a clear solution of benzoic acid by dissolving 0.5g of the benzoic acid sample in about 8 mL of water. 2. 0.5mL of water was added to the flask, while swirling the flask.  This experiment demonstrates the process of recrystallization of benzoic acid from water for the purpose of purifying an organic compound. 1. Simple Distillation Distillation is the joint process of vapourisation and condensation. Theory Benzoic acid is a crystalline solid that has moderate solubility in hot water and low solubility in cold water. The purpose of this experiment is to purify benzoic acid by crystallization. If required, heating can be used to dissolve the benzoic acid. Solubility of benzoic acid in water: 0.21g/ 100mL water at 10oC; 0.27g/ 100mL at 18oC; 2.75g/ 100mL at 80oC; 6.80g/ 100mL at 95oC. Keep the Erlenmeyer flask on a steam bath as you carry out the remainder of the recrystallization process. Benzoic Acid was recrystallized with a 105% recovery using 95% water as the solvent. benzoic acid S-Adenosyl-L-methionine:benzoic acid carboxyl methyltransferase (BAMT) catalyzes the transfer of the methyl group of S-adenosyl-L-methionine (SAM) to the carboxyl group of benzoic acid to make the volatile ester methyl benzoate, one of the most abundant scent compounds of snapdragon, Antirrhinum maj Aim: To purify benzoic acid by recrystallization and to determine the melting point of pure benzoic acid.
This experiment demonstrates the process of recrystallization of benzoic acid from water for the purpose of purifying an organic compound. 1. Simple Distillation Distillation is the joint process of vapourisation and condensation. Theory Benzoic acid is a crystalline solid that has moderate solubility in hot water and low solubility in cold water. The purpose of this experiment is to purify benzoic acid by crystallization. If required, heating can be used to dissolve the benzoic acid. Solubility of benzoic acid in water: 0.21g/ 100mL water at 10oC; 0.27g/ 100mL at 18oC; 2.75g/ 100mL at 80oC; 6.80g/ 100mL at 95oC. Keep the Erlenmeyer flask on a steam bath as you carry out the remainder of the recrystallization process. Benzoic Acid was recrystallized with a 105% recovery using 95% water as the solvent. benzoic acid S-Adenosyl-L-methionine:benzoic acid carboxyl methyltransferase (BAMT) catalyzes the transfer of the methyl group of S-adenosyl-L-methionine (SAM) to the carboxyl group of benzoic acid to make the volatile ester methyl benzoate, one of the most abundant scent compounds of snapdragon, Antirrhinum maj Aim: To purify benzoic acid by recrystallization and to determine the melting point of pure benzoic acid.  Keep adding water in small amounts (several drops at a time from a Pasteur pipette) until all of the benzoic acid is dissolved and the solution is boiling.
Keep adding water in small amounts (several drops at a time from a Pasteur pipette) until all of the benzoic acid is dissolved and the solution is boiling.  titration benzoic nonaqueous adverse effects. Heat the solution for recrystallization benzoic acid results lab report We use this for the purposes of purification of camphor, naphthalene, anthracene, benzoic acid, Iodine and salicylic acid etc containing non-volatile impurities. benzoic purification of benzoic acid.docx - To purify impure sample Impure benzioc acid can be easily purified by means of sublimation/desublimation methods. 1. Heating mantle and variac. benzoic aryl proposed aldehyde oxidoreductase 3. Experiment 4 purification - recrystallization of benzoic acid Preparation of Solution. A Simple Method for the Purification of Benzoic Acid 0.5g impure benzoic acid was placed in a 50mL Erlenmeyer flask.
titration benzoic nonaqueous adverse effects. Heat the solution for recrystallization benzoic acid results lab report We use this for the purposes of purification of camphor, naphthalene, anthracene, benzoic acid, Iodine and salicylic acid etc containing non-volatile impurities. benzoic purification of benzoic acid.docx - To purify impure sample Impure benzioc acid can be easily purified by means of sublimation/desublimation methods. 1. Heating mantle and variac. benzoic aryl proposed aldehyde oxidoreductase 3. Experiment 4 purification - recrystallization of benzoic acid Preparation of Solution. A Simple Method for the Purification of Benzoic Acid 0.5g impure benzoic acid was placed in a 50mL Erlenmeyer flask.