Chemical evaluation. Conversely, liquids of viscosities greater than 25 Pa/s may cause filling problems due to the inability of pumps to fill liquids of this (high) viscosity reproducibly. In the entire operation, youre expected to produce capsules with zero defects. In addition, the presence of sodium lauryl sulphate within the hard gelatin capsule shell is allowed and acts similarly to enhance the uptake of fluid into the capsule shell. Dosing of fill material (The body is filled with the formulation manually using a plastic spatula, and the excess powder is removed). Your perfect and ideal hard gelatin capsule suppliers in China. Powder beds that should demonstrate high cohesion will require greater shearing stresses to initiate and maintain flow. MCQs: Gastroretentive Drug Delivery Systems (GRDDS). These could be powders, pellets, granules, or tablets. Based on all these factors and elements, it is safe to say Hardgel gels capsules take an average of 30 minutes to disintegrate in the body entirely. Hard gelatin capsule shells are fabricated and supplied empty to the pharmaceutical industry by shell suppliers and are then filled in a separate operation. At the end of the filling process, the filled capsules are removed from the turntable. Rectification of capsules (placing empty gelatin capsules on the removable plate with bodies facing downward). Therefore, to prevent repetition, only the basic details of the excipients (including their properties and rationale for use) will be described. The tangent of the angle of repose is frequently referred to as a measure of the internal friction of the powder bed. Tap density measures the volume occupied by the powder bed, both before (ro) and following (rf ) the application of a consolidation stress (generally shaking at a defined rate and for a defined period). Sr. No. Controlling temperature and humidity during the drying process is very critical. The dose is adjusted by the moving piston and a mechanical closure before the piston moves further down to reach to open a channel through which the multiparticulates ow into the capsule body.
When the dosing disk moves the upper end closes und the dosing chamber moves directly above the capsule body to release the pellets. Capsule volumes and typical fill weights for formulations with different tapped densities. A different process is employed in manufacturing. The flow of the powder through the hopper and the homogeneity of the powder mix are maintained by the circular movement of an auger. sunflower, arachis, olive).
So if you need quality and effective empty Hard Gelatin Capsules at a reasonable price, dont hesitate to contact us. Opaque capsules comprise of opacifying agents that make them non-transparent. As drug discovery continues to yield poorly water-soluble molecules, there is an increasing need for formulation techniques that can improve drug solubility. Well, there are so many places where you can buy empty hard gelatin (EHG) capsules. The following excipients are used for the formulation of powder fills: . Gelatin, note, is a compound thats originally derived from the collagen of animal bones.
Here, a wetting agent is used to lower the melting points of the body and caps which are then thermally bonded. So first of, understand that both the hard and softgel capsules are made of gelatin. Filling of multiparticulates by dosator-type machines is achieved by the dosator driving into the pellet bed and sucking in the pellets by vacuum. Which of the following statement is true, ?
So beware of how long you let the pins sit in the gelatin solution.
: Colourants. These are included in liquid fills for hard gelatin capsules to: solubilise the therapeutic agent within the solvent stabilise the suspended therapeutic agent enhance the dissolution of poorly water-soluble therapeutic agents within the gastrointestinal tract. As detailed above, there are three main classes of fill that are employed in hard gelatin capsules, namely powders and liquids/semisolids. Typically the flow properties and the packing properties of the powders are assessed using the following techniques: . The production of coloured capsules requires the addition of the appropriate colour and opacifying agent (e.g. The body has a slightly lower diameter than the cap and fits inside the cap. This can be a tamping pin or Dosator type capsule filling machine, either of which can be automatic, semi-automatic, or manual. You can employ automated sorting machines for this, or go the manual way by conducting visual and physical checks. This makes them unsuitable for a market with varying demands. You also have unique Blog one wont see in other blogs. Color is the most fundamental part of a drugs personality. As the viscosity is lowered the capsule thickness will decrease. Then, transfer the mixture to a stainless steel service tank and add dyes and water. Tablets for capsule filling are normally film-coated to prevent dust generation and are sized so that they can fall freely into the capsule body but without turning on their sides. Besides confirmation of identity and determination of quality and purity the term drug evaluation also covers detection and determination of thetype of adulteration present. This step also notes, is not mandatory. Other ingredients are also used to give the capsules a positive binding action, desired shade, and color, etc. While evaluating by this method there arecertain restrictions like changes in shape & size of drugs during drying & packing so it is Difficult to the study the drug by organoleptic evaluation. Hard gels should be perfect concerning shape, size, appearance, color, texture, taste, and order. Here, gently moving air which is accurately controlled for temperature and humidity remove the moisture from the capsule halves. Only disintegrates in the presence of digestive fluids. This softens the capsule and, following heating to 45C, the interface fuses to produce a seal.
glyceryl monostearate). After completing the test, capsules move to the packaging stage, where they get packed conventionally. Acceptable powder flow also requires that clumping of the powders does not occur. Once here, lower the machine plate to the gelatin bath.
Note: If hard gelatin capsules cannot be used because of any formulation, preference, or cultural reason, alternative materials such as polymer-based shells or hypromellose may be used. Simple Description of the Hard Gelatin Capsule Filling Process. fatty acid esters (e.g. Hard gelatin capsules are composed of two halves, termed the cap and the body. To optimize the process conditions such as method of agitation, time of screening, feed rate etc. Following dissolution of the polymer a vacuum is then applied to the mixing vessel to remove entrapped air. There are several general properties of powders that should be recognized when formulating these as capsule fills, as follows: The particle size distributions, of the various components of the powder mix (including the therapeutic agent) should be similar both to ensure homogeneous mixing and to minimize segregation. All Rights Reserved. But gelatin is not all that is used to produce the two-piece gelatin capsules. These are based on the color and opaqueness of the capsule shell. A major concern in the choice and hence formulation of solvents for liquid fill formulations is the effect of the formulation on the stability of the capsule. For this purpose the chosen dye is added at the required concentration into the heated gelatin solution. Hard gelatin capsules can also be hand-filled one at a time, as done in a compounding pharmacy. The dosator device uses an empty tube that dips into powder bed, which is maintained at a height approximately two-fold greater than the desired length of the plug. The process of encapsulation requires you to use an encapsulation machine, also known as a capsule filling machine. Or if youre buying from a Hard Gelatin Capsule supplier, make sure that the supplier is reputable. From the above, the significance of Hard Gelatin Capsules in the pharmaceutical industry is quite clear.
That is, from the raw material selection stage. First off, Hard Gelatin Capsules are used in several pharmaceutical, food, and cosmetic industries. Therefore, these Hard Gelatin Capsules are readily available in different sizes, colors, prints, and dimensions.
Replacement of caps/ closing capsule shells and. They are a well-established dosage form that provides solutions to many of todays drug delivery and nutraceutical formulation challenges. Remember that the body of each capsule has a defined volume. The formulation considerations for these categories are considered separately in the following sections. Color, as Ive also said, is a great way to distinguish one brand from another. Also called as, Sifting. Capsules formulated to give modified-release patterns are often filled with granules or coated pellets using machines adapted from powder use. The dosing disk method can be adapted in a way that the filled dosing chambers are not tamped and the filled dosing chamber is transferred above the capsule body, where a sliding plate opens the chamber to release the multiparticulates. If the water content is lower than the above specification, the capsule shell will become brittle and will crack when exposed to the appropriate stress. The opaque nature of the capsules will help protect the contents from the effects of light, i.e. In the capsule filling industry, Hard Gelatin Capsules are known to be the least costly. This understanding is critical because the capsule youre looking to produce is meant for human consumption. Of course, you need to have the right know-how and machinery to do this. elongated size 00 capsules (00el), elongated size 0 capsules (0el), elongated size 1 capsules (1el), elongated size 2 capsules (2el) etc. Control of the viscosity is important as this regulates the thickness of the capsule (generally circa 100 m). Fundus ? Official Standards of Sieves: Indian Pharmacopoeia has prescribed standards for powders for pharmaceutical purposes. Taste of clove is pungent. If the measured angle exceeds 50, the flow properties of the powder are poor. To test the efficiency of a size reduction equipment or process.
Once the trimming comes to its end as per the requirements, the machine automatically joins the two halves into blocks. It is preferable that the particle size distribution of the powder blend is both monomodal and exhibits low polydispersity (low standard deviation) to ensure predictable and reproducible flow during the filling process. Generally, the disintegration time ranges from 3-13 minutes, depending on the size of the capsules. ? If youre into capsule filling and are looking for a versatile capsule to use, Hard Gelatin Capsules are a perfect choice. ? Hard gels, basically can satisfy your need and that of your customers adequately. So with your formulation ready and dosing weight established, it is easy to define the most appropriate capsule size. And, by knowing the density of your formulation, you can easily establish the dosage of each capsule. The first step to estimating the optimal capsule size for a given product is to determine the density of the formulation using tapped density for powders and bulk density for pellets, minitablets, and granules.
The storage condition of Hard Gelatin Capsules can greatly affect its quality, so, you should maintain them properly. SaintyCo is a professional manufacturer of hard gelatin capsules with the highest quality. The filling of hard gelatin capsules is an established technology, with equipment available ranging from that for very small-scale manual filling (e.g., Feton capsule filling machine), through intermediate-scale semi-automatic filling to large-scale fully automatic filling. Unfortunately, the many processes involved in manufacturing tend to expose hard gels to several defects that include; Note that these defects can sometimes cause severe problems. Examples of excipients that are employed in the formulation of powder fills include: Lubricants are used to reduce the interaction of the powders with the metal dossator and/or other metal components of the filling machine, whereas glidants are used to lower the interparticle attraction, thereby reducing clumping and aiding powder flow. The pins are then advanced through a series of air dryers in which air of the required humidity is passed across the surface of the gelatin film. Multiparticulate dosator capsule filling principle. Gelatin forms strong and transparent gels that are easily digestible, and are soluble in hot water. Note that printing can be done in a different form that helps in capsule identification, dosage information, as well as promotion. Liquid/semisolid fills for hard gelatin capsules may be subdivided into various categories: Examples of the types of liquids that are commonly used in this category include: , vegetable oils (e.g. length of thecinnamon quill is 1 meter but mostly. To help you meet the different demands of your market, we provide a range of capsule sizes for your choice. It is based on various steps and concerns that were going to discuss in this section. The various stages of this are as follows: Preparation of the gelatin solution Initially a concentrated solution of gelatin is prepared (circa 3540% w/w) in demineralised hot water with stirring. Following adsorption of the gelatin solution onto the surface of the pins, the bar containing the pins is removed and rotated. The pins (one set for the production of the cap and one for the body of the capsule) are dipped into a pan that contains the heated gelatin solution (maintained at 3545C). As noted earlier in this guide, the process of capsules manufacturing involves a lot of processes. Capsule filling, also known as encapsulation, is a process of enclosing certain medicines inside empty capsules, allowing them to be taken orally. Besides, Machines and equipment from SaintyCo must undergo a rigorous quality inspection and validation process. As stated previously, the equilibrium moisture content of hard gelatin capsules should be 1316% to ensure that the capsule exhibits the optimal mechanical properties. Once the manufacturing and printing of capsules come to its end, capsules transfer to the testing cell. The filing process varies depending on the type of capsule filler used. This is more of a book than a Blog. Note that at this stage, water (hot) should only be used to adjust the viscosity of the mixture. Their inclusion is particularly important if the formulation contains significant concentrations of hydrophobic components, e.g. Choose SaintyCo now for the best and most authentic hard gelatin capsules producer. Apart from this, there are many other elements like the quality of coloring and technology used in manufacturing. A hard gelatin capsule, also known as a dry-filled capsule or two-piece capsule, is in a solid dosage form. ?
document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); (adsbygoogle = window.adsbygoogle || []).push({}); Copyright 2022 Pharmapproach Limited. Another primary reason why you should use Hard Gelatin Capsules is that theyre effortless to fill. Manufacturing and filling is done using the same machine, as part of a single process, Hard gel capsules usually are cylindrical.
If the viscosity is lower than this range, there will be a loss in the capsule contents due to splashing of the liquid from the capsule during filling. Examples of the types of liquids that are commonly used in this category include: polyethylene glycols (PEGs) that are solid at room temperature but will liquefy upon heating (e.g. 7 Differences between Oil-in-Water and Water-in-Oil Emulsions, Preformulation Studies: Bulk Characterization, Application of Ultrasound to the Pharmaceutical Industry, WHO Eastern Mediterranean Region Country Offices, National Agency for Food & Drugs Admin & Control, Journal of Pharmaceutical Development and Industrial Pharmacy, Poloxamers, Lecithin, PEG esters (e.g., Gelucir 44/14; 50/13; Labrafil). The goodness of the Hard Gelatin Capsules is that you can make or get them in a variety of specifications. Gelatin band sealing. To achieve rapid drug release the powder fill should be readily dispersed within the gastrointestinal contents, a feature that is enhanced by the presence of surface-active agents of high hydrophilelipophile balance, e.g. You need to be sure that the final capsules you have, whether self-manufactured or bought, pass the requisite quality control tests. If filling a small batch of capsules, cleaning can be done manually by rubbing a clean cloth or gauze over the capsules. The joined blocks are then shoved into the conveyor belt, which in turn transfers them out to a container. Powder filling may require a soft compact (plug) formation depending on the formulation weight and capsule fill volume. Notify me of follow-up comments by email. In general, powder formulations for inclusion within hard gelatin capsules should exhibit the following properties: Good flow properties. SCHDULE Details A Application for the licenses, issue and renewal of licenses, for sending memoranda under the act B Rate of fee for test or analysis by the Central Drugs Laboratory or the Government Analyst. The IP 1996 specifies five grades of powders. And to achieve this, you need to regulate your inspection system. The only challenge is in ascertaining the reliability of the EHG capsule supplier as this is not easy. highermolecular-weight PEGs) liquid polyoxyethylene-polyoxypropylene block co-polymers (Pluronics).
Multiparticulate volumetric chamber filling principle for multiple products, Pellet dosing based on volumetric filling by the moving dosing disk principle. The formulation considerations for both of these strategies are individually discussed below.
Whatever means you use, ensure that you detect and remove any defective capsules before packaging. In addition, the diluents may offer additional properties, most notably their flow properties and their ability to undergo compression. Chemistry Problem Solver: How to Become One? Once the drying process comes to its end, the pin plate enters the table section of the machine. If required, the bonding between the particles within the plug may be enhanced by the application of a compression pressure. If you are searching for permanent businesses of hard gelatin capsules, SaintyCo can be your best partner. Once you achieve the targeted level of viscosity, proceed to dip. They cause stability problems. During the capsule filling unit operation, the body is filled with the drug substances and the shell is closed by bringing the body and the cap together. Hard gelatin capsules are manufactured using a dip-coating method and the various stages involved are as follows: Step 1: Preparation of the gelatin solution (dipping solution), Step 2: Dip-coating the gelatin solution on to metal pins (moulds), Step 4: Drying of the gelatin-coated pins, Step 6: Joining of the trimmed capsule shell, Read More On: Manufacture of Hard Gelatin Capsules. A hard gelatin capsule is a solid dosage form that is also referred to as a dry-filled capsule or two-piece capsule. sodium lauryl sulphate.
In this step, you need to print the capsules as per the requirement. The machine used to manufacture capsules consists of two sets of bars, each containing a series of pins (aligned in columnar formation) that have been lubricated prior to use. SaintyCosDosator Type Ccapsule Filling Machines. 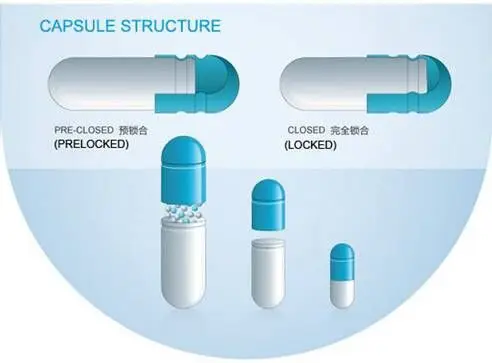 After this, the top plate of the machine will drop back into position, where the caps will be placed back onto the filled bodies. Finished product. The degree of this effect will be greater the smaller the capsule size and the larger the particle diameter. The limit test for Chlorides measures, ? The biggest concern in the production of Hard Gelatin Capsule filling machine usually lies in how to control quality. For drugs that need to be released at specific times and points in the body, target release pellets are often used. The ratio of the density of the powder bed before to that after shaking is referred to as a Hausner ratio. soft gelatin, plasticizer, sugars, preservatives, colorings, opacifier, etc. We can provide different types and kinds of hard gelatin capsules that are perfect for your requirements. As a quality control tool for the analysis of raw materials such as griseofulvin and aspirin. Due to this, it is essential to properly check for defects before dispatching capsules, whether empty or filled. Raw material used in the process. In this method, a dilute solution of gelatin is applied to the center of the capsule (between the two halves) which, once dried, produces a hermetic seal. The ratio of gelatin and plasticizer is 1:0.4, The ratio of gelatin to plasticizer is 1:0.8, Plasticizers to give the gelatin the desired flexibility and elasticity, Edible colorings to achieve the right choice of color ( optional), Demineralized water for initial gelatin preparation, Glycerin to decrease the capsules hardness, A high water content that makes it unsuitable for use with liquid formulations. These are made of only hard gelatin, colorings, plasticizer and titanium dioxide. Clear Hard Gelatin Capsules are the ideal choice when you want to show off the color and texture of the contents in the capsules. Prominently, there are four types of Hard Gelatin Capsules. The technique provides an indication of the flow properties of the powder and, specifically, is a measure of cohesion within the powder mass. And for this to happen, the right formulation has to be made. Note that a typical Hardgel capsule has between 12% to 15% water content. As a general rule, the formulation should have a viscosity of between 50 and 1000 Centipoise (cP) (although formulations of much higher viscosity can be suitable for manufacturing) and should not exceed 70 C. lubricants and glidants. It is permissible to formulate hard gelatin capsules with sodium lauryl sulphate ( 0.15% w/w) as an included component to increase the wetting properties of the capsule shell following contact with an aqueous solution (and hence enhance the dissolution properties of the formulation contained within the capsule). A second plate then opens and closes at the bottom of the chamber to fill the metered pellet dose into the capsule. Theyre manufactured using different machines, for shell production and encapsulation. Turbidity due to Silver Chloride. During manufacture of the dosage form, the formulation is filled into the body (using a range of different mechanical techniques) and the cap is pushed into place. taste of fennel is sweet. At this point, the top plate of the filler is lifted to separate the cap from the body of the capsules. Thus the difference between these two types of capsules lies in the design and manufacturing technology. Compared to other types of capsules, hard gels tend to have a longer shelf life with minimum maintenance process. Knowing the difference between the two might help you make the right choice when looking to procure capsules for your project/s. Hydroalcoholic solvent seal. Most encapsulators used for this purpose have a mechanical probe that is inserted into the capsule to check that the correct number of tablets has been transferred. So make sure that you use a machine that can do this precisely. Hard gelatin capsules also contain 1216% water, but the water content can vary, depending on the storage conditions. e.g. The final capsules should exhibit a water content of 1316% w/w. One question that always comes to mind when thinking of capsules is what material is used to make the shells. Once coated, let the plate rise to the upper deck, which should release the capsule and its body to get set on the pins. For instance, a cracked capsule will have contents spilling out and causing unsafe effects to the end-user. And that he follows the set industry standards on capsule production and formulation.
After this, the top plate of the machine will drop back into position, where the caps will be placed back onto the filled bodies. Finished product. The degree of this effect will be greater the smaller the capsule size and the larger the particle diameter. The limit test for Chlorides measures, ? The biggest concern in the production of Hard Gelatin Capsule filling machine usually lies in how to control quality. For drugs that need to be released at specific times and points in the body, target release pellets are often used. The ratio of the density of the powder bed before to that after shaking is referred to as a Hausner ratio. soft gelatin, plasticizer, sugars, preservatives, colorings, opacifier, etc. We can provide different types and kinds of hard gelatin capsules that are perfect for your requirements. As a quality control tool for the analysis of raw materials such as griseofulvin and aspirin. Due to this, it is essential to properly check for defects before dispatching capsules, whether empty or filled. Raw material used in the process. In this method, a dilute solution of gelatin is applied to the center of the capsule (between the two halves) which, once dried, produces a hermetic seal. The ratio of gelatin and plasticizer is 1:0.4, The ratio of gelatin to plasticizer is 1:0.8, Plasticizers to give the gelatin the desired flexibility and elasticity, Edible colorings to achieve the right choice of color ( optional), Demineralized water for initial gelatin preparation, Glycerin to decrease the capsules hardness, A high water content that makes it unsuitable for use with liquid formulations. These are made of only hard gelatin, colorings, plasticizer and titanium dioxide. Clear Hard Gelatin Capsules are the ideal choice when you want to show off the color and texture of the contents in the capsules. Prominently, there are four types of Hard Gelatin Capsules. The technique provides an indication of the flow properties of the powder and, specifically, is a measure of cohesion within the powder mass. And for this to happen, the right formulation has to be made. Note that a typical Hardgel capsule has between 12% to 15% water content. As a general rule, the formulation should have a viscosity of between 50 and 1000 Centipoise (cP) (although formulations of much higher viscosity can be suitable for manufacturing) and should not exceed 70 C. lubricants and glidants. It is permissible to formulate hard gelatin capsules with sodium lauryl sulphate ( 0.15% w/w) as an included component to increase the wetting properties of the capsule shell following contact with an aqueous solution (and hence enhance the dissolution properties of the formulation contained within the capsule). A second plate then opens and closes at the bottom of the chamber to fill the metered pellet dose into the capsule. Theyre manufactured using different machines, for shell production and encapsulation. Turbidity due to Silver Chloride. During manufacture of the dosage form, the formulation is filled into the body (using a range of different mechanical techniques) and the cap is pushed into place. taste of fennel is sweet. At this point, the top plate of the filler is lifted to separate the cap from the body of the capsules. Thus the difference between these two types of capsules lies in the design and manufacturing technology. Compared to other types of capsules, hard gels tend to have a longer shelf life with minimum maintenance process. Knowing the difference between the two might help you make the right choice when looking to procure capsules for your project/s. Hydroalcoholic solvent seal. Most encapsulators used for this purpose have a mechanical probe that is inserted into the capsule to check that the correct number of tablets has been transferred. So make sure that you use a machine that can do this precisely. Hard gelatin capsules also contain 1216% water, but the water content can vary, depending on the storage conditions. e.g. The final capsules should exhibit a water content of 1316% w/w. One question that always comes to mind when thinking of capsules is what material is used to make the shells. Once coated, let the plate rise to the upper deck, which should release the capsule and its body to get set on the pins. For instance, a cracked capsule will have contents spilling out and causing unsafe effects to the end-user. And that he follows the set industry standards on capsule production and formulation.  The fill weight for liquids is calculated by multiplying the specific gravity of the liquid by the capsule body volume multiplied by 0.9. The settings of the spring-loaded piston control the volume of powder that enters the tube. Once the formulation is ready, you need to choose the right capsule size to fill. As the filling is performed according to volume, it is important that the packing of the particles is also reproducible, as variations in this property will result in differences in the mass of powder filled into each capsule. Here are more details on why you should use Hard Gelatin Capsules. Before that, I need you to understand that the complete manufacturing process of Hard Gelatin Capsules is quite tricky. Due to the tight fit between the two halves, separation of the cap and body does not normally occur under normal storage conditions or in clinical use. Hard gelatin capsule shell is composed largely of gelatin. Moreover, the capsules have no taste making them an excellent option to mask the taste and smell of bitter medicine and herbal dosages. This is the point where you ensure the quality of the capsules youve manufactured. You are welcome. Accordingly, degree of coarseness or fineness is expressed with reference to the nominal aperture size of sieve through which powder is able to pass. And here, your choice will depend on your unit dose requirements as well as the formulation youre using. Here, the capsules are divided into two equal or roughly equal sizes which are then stripped off the pins. needle shape). Finally, as the filling process may require plug formation (within the dossator), the powder bed should be compressible. Sealing of hard gelatin capsules may also be performed using two further methods: 1. The size of hard gelatin capsule selected for use is determined by requirements of the formulation, including the dose of the active ingredient and the density and compaction characteristics of the drug and other components.
The fill weight for liquids is calculated by multiplying the specific gravity of the liquid by the capsule body volume multiplied by 0.9. The settings of the spring-loaded piston control the volume of powder that enters the tube. Once the formulation is ready, you need to choose the right capsule size to fill. As the filling is performed according to volume, it is important that the packing of the particles is also reproducible, as variations in this property will result in differences in the mass of powder filled into each capsule. Here are more details on why you should use Hard Gelatin Capsules. Before that, I need you to understand that the complete manufacturing process of Hard Gelatin Capsules is quite tricky. Due to the tight fit between the two halves, separation of the cap and body does not normally occur under normal storage conditions or in clinical use. Hard gelatin capsule shell is composed largely of gelatin. Moreover, the capsules have no taste making them an excellent option to mask the taste and smell of bitter medicine and herbal dosages. This is the point where you ensure the quality of the capsules youve manufactured. You are welcome. Accordingly, degree of coarseness or fineness is expressed with reference to the nominal aperture size of sieve through which powder is able to pass. And here, your choice will depend on your unit dose requirements as well as the formulation youre using. Here, the capsules are divided into two equal or roughly equal sizes which are then stripped off the pins. needle shape). Finally, as the filling process may require plug formation (within the dossator), the powder bed should be compressible. Sealing of hard gelatin capsules may also be performed using two further methods: 1. The size of hard gelatin capsule selected for use is determined by requirements of the formulation, including the dose of the active ingredient and the density and compaction characteristics of the drug and other components. 