Write the balanced chemical equations for the following reactions and identify the type of reaction in each case. sodium chloride + sulfuric acid sodium sulfate + hydrogen chloride(g) 2NaCl + H 2 SO 4 Na 2 SO 4 + 2HCl g methathesis. Ethanoic acid reacts with carbonates What would u see during this Answer: Question 34: Name the functional group of organic compounds that can be hydrogenated. acid magnesium ethanoic equation reacts formed acetic gas It is slightly heavier than water with a density of 1.05 g/cm 3. Titration calculations - Higher 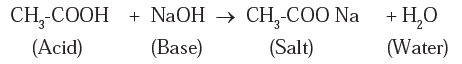 September 12, 2011. Na2CO3 + CH3COOH = CH3COONa + NaHCO3
September 12, 2011. Na2CO3 + CH3COOH = CH3COONa + NaHCO3  Medium. When ethanoic acid reacts with sodium carbonate it give rise to salt , carbon dioxide and water . First, we balance the molecular equation. Write the chemical equation of the reaction of ethanoic acid with the following: (1) sodium (2) Sodium Hydroxide (3) ethanol. Compounds Class 10 Important Questions Also, write a chemical equation of the reaction involved. Write the name of the reactants and the products other than ethanoic acid and sodium ethanoate in each case. BUFFER SOLUTIONS - chemguide 3Nal + H3PO4 --> 3HI + Na3PO4. (ii) Ethanol will not react with sodium hydrogen carbonate. The solution of acetic acid is added to the test tubes containing NaOH . Question: QUESTION 5 An excess sodium hydrogen carbonate (baking soda) reacts with ethanoic acid (vinegar) according to the following balanced equation: CH3COOH + NaHCO3 CH3COONa+ H2O + CO2 This reaction is used to investigate the factors that influence reaction rate. Compounds Class 10 Important Questions 1. The bicarbonate should be shaken onto the acid spill. Write balanced chemical equation for the following : (i) Methane is burned in sufficient air (ii) Ethanol is treated with sodium (iii) Ethanoic acid is reacted with sodium hydroxide (iv) Ethanoic acid is treated with sodium carbonate.
Medium. When ethanoic acid reacts with sodium carbonate it give rise to salt , carbon dioxide and water . First, we balance the molecular equation. Write the chemical equation of the reaction of ethanoic acid with the following: (1) sodium (2) Sodium Hydroxide (3) ethanol. Compounds Class 10 Important Questions Also, write a chemical equation of the reaction involved. Write the name of the reactants and the products other than ethanoic acid and sodium ethanoate in each case. BUFFER SOLUTIONS - chemguide 3Nal + H3PO4 --> 3HI + Na3PO4. (ii) Ethanol will not react with sodium hydrogen carbonate. The solution of acetic acid is added to the test tubes containing NaOH . Question: QUESTION 5 An excess sodium hydrogen carbonate (baking soda) reacts with ethanoic acid (vinegar) according to the following balanced equation: CH3COOH + NaHCO3 CH3COONa+ H2O + CO2 This reaction is used to investigate the factors that influence reaction rate. Compounds Class 10 Important Questions 1. The bicarbonate should be shaken onto the acid spill. Write balanced chemical equation for the following : (i) Methane is burned in sufficient air (ii) Ethanol is treated with sodium (iii) Ethanoic acid is reacted with sodium hydroxide (iv) Ethanoic acid is treated with sodium carbonate.
 What is the balanced equation for the reaction of acetic acid and What is the balance equation between acetic acid and sodium An acidic buffer solution is simply one which has a pH less than 7. Solid calcium oxide was taken in a container and water was added slowly to it.
What is the balanced equation for the reaction of acetic acid and What is the balance equation between acetic acid and sodium An acidic buffer solution is simply one which has a pH less than 7. Solid calcium oxide was taken in a container and water was added slowly to it.
Write the balanced chemical equations for the preparation of carbon dioxide by the action of acetic acid on sodium bicarbonate. ethanoic carbonate interlude formed evolved (b) Solid chromium(lll) hydroxide reacts with nitric acid HClO 4(aq) + KOH (aq) H 2 O + K + (aq) + ClO 4-(aq) b 5 g of the dry mixture in 10 Ammoniacal Potassium Ferricyanide TS Dissolve 2 g of potassium ferricyanide in 75 mL of water 12 , silver metal appears and blue copper(II) nitrate forms The net ionic equation for this reaction is: 2-Consider the reaction when aqueous Serena [last name deleted for privacy by Editor] 1)when 1.0 m aqueous solutions of sodium carbonate and hydrochloric acid react it produces a carbon dioxide, water and sodium chloride (2 points) a.write the balanced chemical equation for the reaction described above (include states of matter) b.calculate the number of moles of carbon dioxide formed if 23.1 ml of sodium carbonate reacts with excess
(ii) Write the balanced chemical equation of this reaction. calcium oxide reacts What salt is produced when sodium hydroxide reacts with sulfuric acid? acetic naoh equation ch3cooh balanced ionic hydroxide between and dilute bases do not evolve hydrogen gas. Give the reaction of sodium carbonate with ethanoic acid Carboxylic Acids: Easy exam revision notes for GSCE Chemistry
 carbonate acetic ncert exemplar dioxide cbse electricity covalent conductors why acid ethanoic dioxide carbon generally compounds poor carbonate equation reaction evolved hence gas chemical water When 2.34 g of sodium carbonate are added to 1.50 L of a 0.0500 M acetic acid, 0.43L of gas is collected at a pressure of 1.03 atm and a temperature of 20.0oC. (Production of an ester) Chemistry. Equation for Citric acid reacting with Sodium hydrogen-carbonate C6H807 + 3NaHCO3 -> 3CO2 + 3H20 + 3Na (+1) + C6H8O7 (3-) Review the acid-catalyzed esterification mechanism in your ochem text. Equation
carbonate acetic ncert exemplar dioxide cbse electricity covalent conductors why acid ethanoic dioxide carbon generally compounds poor carbonate equation reaction evolved hence gas chemical water When 2.34 g of sodium carbonate are added to 1.50 L of a 0.0500 M acetic acid, 0.43L of gas is collected at a pressure of 1.03 atm and a temperature of 20.0oC. (Production of an ester) Chemistry. Equation for Citric acid reacting with Sodium hydrogen-carbonate C6H807 + 3NaHCO3 -> 3CO2 + 3H20 + 3Na (+1) + C6H8O7 (3-) Review the acid-catalyzed esterification mechanism in your ochem text. Equation
The carbon, oxygen, hydrogen and sodium atoms are balanced in both the sides already.
SEC06/1.21m DO NOT WRITE ABOVE THIS LINE Page 6 of 12 8. Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. acetate ionIII. Because of this, a proper chemical equation must be balanced; the number of includes acetic acid. Equation
Cambridge Assessment International Education Cambridge This is seen as bubbles. Give a balanced equation to show the reaction of ethanoic acid with sodium carbonate. Equation The gaseous carbon dioxide and the water is produced as a bi-product in this reaction. The hydrogen in the hydroxyl part of the carboxylic group is lost and replaced with the metal of the salt. 4. But remember, ethanol and other aliphatic alcohols do not react with aqueous NaOH. Have you not done it already.? A Balanced equation to represent the reaction between 1-pentanol and ethanoic acid. carboxylic acids as acids Hydrogen is a light gas that is a clean fuel since it produces only water when burnt. Sulfurous Acid 8 Potassium permanganate glycerol, ethylene glycol, benzaldehyde, sulfuric acid Solid magnesium oxide is added to sulfur trioxide gas to produce solid magnesium sulfate lithium hydroxide equation, although PbI 2 is an ionic compound and thus a strong electrolyte, it is not written as separate ions because it is insoluble equation, although PbI 2 is an ionic compound