Other nylons include copolymerized dicarboxylic acid/diamine products that are not based upon the monomers listed above.
chemistry chemically lon yields hydrogen [19]:145147[15] Realizing the danger of claims such as "New Hosiery Held Strong as Steel" and "No More Runs", DuPont scaled back the terms of the original announcement, especially those stating that nylon would possess the strength of steel. This gives it almost the same carbon footprint as wool, but with greater durability and therefore a lower overall carbon footprint.[75]. Close it when you are ready to come back here. [93][6]:514, Molded nylon is used in hair combs and mechanical parts such as machine screws, gears, gaskets, and other low- to medium-stress components previously cast in metal.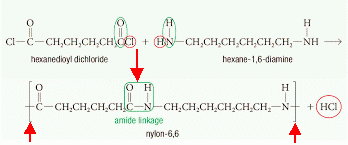
 chemistry [6]:53, In the mid-1940s, classical guitarist Andrs Segovia mentioned the shortage of good guitar strings in the United States, particularly his favorite Pirastro catgut strings, to a number of foreign diplomats at a party, including General Lindeman of the British Embassy. The characteristic features of nylon 6,6 include: On the other hand, nylon 6 is easy to dye, more readily fades; it has a higher impact resistance, a more rapid moisture absorption, greater elasticity and elastic recovery. [19]:138139
chemistry [6]:53, In the mid-1940s, classical guitarist Andrs Segovia mentioned the shortage of good guitar strings in the United States, particularly his favorite Pirastro catgut strings, to a number of foreign diplomats at a party, including General Lindeman of the British Embassy. The characteristic features of nylon 6,6 include: On the other hand, nylon 6 is easy to dye, more readily fades; it has a higher impact resistance, a more rapid moisture absorption, greater elasticity and elastic recovery. [19]:138139  organic nylon substances chemistry important hence polymeric chains hydrogen bond between
organic nylon substances chemistry important hence polymeric chains hydrogen bond between
 [17] Also, consumers became concerned about environmental costs throughout the production cycle: obtaining the raw materials (oil), energy use during production, waste produced during creation of the fiber, and eventual waste disposal of materials that were not biodegradable. Type 6,6 Nylon 101 is the most common commercial grade of nylon, and Nylon 6 is the most common commercial grade of molded nylon.
[17] Also, consumers became concerned about environmental costs throughout the production cycle: obtaining the raw materials (oil), energy use during production, waste produced during creation of the fiber, and eventual waste disposal of materials that were not biodegradable. Type 6,6 Nylon 101 is the most common commercial grade of nylon, and Nylon 6 is the most common commercial grade of molded nylon. 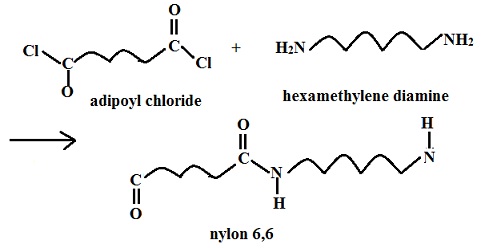 Proteins,
Proteins, 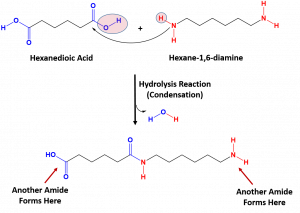
 [6]:209, Nylon powders are used to powder coat metals. fibers. America's Textile Reporter referred to 1951 as the "Year of the blending of the fibers". Incinerating nylons to recover the high energy used to create them is usually expensive, so most nylons reach the garbage dumps, decaying slowly. In keeping with this naming convention, "nylon6,12" or "PA 612" is a copolymer of a 6C diamine and a 12C diacid. ", "Process for producing 1,9-nonanedial US 4510332 A", "Preparation of xylylenediamines US 2970170 A", "Ajinomoto and Toray to Conduct Joint Research on Biobased Nylon", "Durethan is the trade name for our range of engineering thermoplastics based on polyamide 6 and polyamide 66", "Polyamide Resins for an Extreme World Flagship Rilsan PA11 and Complementary Resins & Alloys", "Stanyl Polyamide 46: Driving change in automotive", "zytel - PA6, PA610, PA612, PA66 - dupont", "Approximate Time it Takes for Garbage to Decompose in the Environment", "Recycling nylon is good for the planet so why don't more companies do it? [18][19]:141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers. [7], Nylon was the first commercially successful synthetic thermoplastic polymer.
[6]:209, Nylon powders are used to powder coat metals. fibers. America's Textile Reporter referred to 1951 as the "Year of the blending of the fibers". Incinerating nylons to recover the high energy used to create them is usually expensive, so most nylons reach the garbage dumps, decaying slowly. In keeping with this naming convention, "nylon6,12" or "PA 612" is a copolymer of a 6C diamine and a 12C diacid. ", "Process for producing 1,9-nonanedial US 4510332 A", "Preparation of xylylenediamines US 2970170 A", "Ajinomoto and Toray to Conduct Joint Research on Biobased Nylon", "Durethan is the trade name for our range of engineering thermoplastics based on polyamide 6 and polyamide 66", "Polyamide Resins for an Extreme World Flagship Rilsan PA11 and Complementary Resins & Alloys", "Stanyl Polyamide 46: Driving change in automotive", "zytel - PA6, PA610, PA612, PA66 - dupont", "Approximate Time it Takes for Garbage to Decompose in the Environment", "Recycling nylon is good for the planet so why don't more companies do it? [18][19]:141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers. [7], Nylon was the first commercially successful synthetic thermoplastic polymer.
4,000 pairs of stockings were available, all of which were sold within three hours. [19]:146147 Nylon was thus domesticated,[19]:151152 and attention shifted to the material and consumer aspect of the fiber with slogans like "If it's nylon, it's prettier, and oh! [29] Although nylon stockings already made before the war could be purchased, they were generally sold on the black market for as high as $20.[27]. During World War II, almost all nylon production was diverted to the military for use in parachutes and parachute cord.
In the second case (so called AA), the repeating unit corresponds to the single monomer.
of carbon atoms, each being six carbon atoms long.
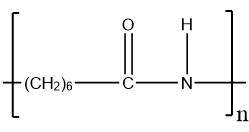
The image below is of the caprolactam pdb model you can view by clicking here or on the image. For example, some fully aromatic nylons (known as "aramids") are polymerized with the addition of diacids like terephthalic acid ( Kevlar, Twaron) or isophthalic acid ( Nomex), more commonly associated with polyesters. [7] Some of the terpolymers based upon nylon are used every day in packaging. from the monomers adipoyl chloride and hexamethylene diamine. Nylon is also found in clothing such as windbreakers, and (ahem) lingerie, but also in other places, [17]:101 In 1941, a second plant was opened in Martinsville, Virginia, due to the success of the fabric. 6,6, because each repeat unit of the polymer chain has two stretches
fishing line and trimmer line, plus it's used for some "plastic" screws and The planar amide (-CO-NH-) groups are very polar, so nylon forms multiple hydrogen bonds among adjacent strands. The first example of nylon, (nylon 66), was synthesized using diamines on February 28, 1935, by Wallace Hume Carothers at DuPont's research facility at the DuPont Experimental Station.
Wallace Carothers at DuPont patented nylon66 using amides. Most nylons are made from the reaction of a dicarboxylic acid with a diamine (e.g. [18], Another added bonus to the campaign was that it meant reducing silk imports from Japan, an argument that won over many wary customers. [17]:101 Sales of nylon stockings were strong in part due to changes in women's fashion. 2, 1978) explained that the name was originally intended to be "No-Run" ("run" meaning "unravel"), but was modified to avoid making such an unjustified claim. nylon polycarbonate poly chemistry tutorial fig Nylon was even mentioned by President Roosevelt's cabinet, which addressed its "vast and interesting economic possibilities" five days after the material was formally announced. Data published by PlasticsEurope indicates for nylon 66 a greenhouse gas footprint of 6.4kg CO2 equivalent per kg, and an energy consumption of 138 kJ/kg. Nylon510 can have coordinated runs of 5 and 8 carbons. DuPont, skeptical of the idea, agreed to supply the nylon if Augustine would endeavor to develop and produce the actual strings. [24], An important part of nylon's popularity stems from DuPont's marketing strategy.
nylon polycarbonate poly chemistry tutorial fig Nylon was even mentioned by President Roosevelt's cabinet, which addressed its "vast and interesting economic possibilities" five days after the material was formally announced. Data published by PlasticsEurope indicates for nylon 66 a greenhouse gas footprint of 6.4kg CO2 equivalent per kg, and an energy consumption of 138 kJ/kg. Nylon510 can have coordinated runs of 5 and 8 carbons. DuPont, skeptical of the idea, agreed to supply the nylon if Augustine would endeavor to develop and produce the actual strings. [24], An important part of nylon's popularity stems from DuPont's marketing strategy.
Nylon is clear and colorless, or milky, but is easily dyed. materials, like parachutes and ropes. Either way, be sure to close the new windows that open up with the 3D model in it when you are ready to come back here. [15] The Lunar Flag Assembly, the first flag planted on the moon in a symbolic gesture of celebration, was made of nylon. Since the products were not really run-proof, the vowels were swapped to produce "nuron", which was changed to "nilon" "to make it sound less like a nerve tonic". The second image is of the diamine monomer. When extruded into fibers through pores in an industry spinneret, the individual polymer chains tend to align because of viscous flow. The image on the left is of the 3D model of nylon 6,6; on the right is nylon 6. Segovia found that although the strings produced a clear sound, they had a faint metallic timbre which he hoped could be eliminated. Although scientists asserted that cadaverine was also extracted by heating coal, the public often refused to listen. For copolymers the comonomers or pairs of comonomers are separated by slashes: The term polyphthalamide (abbreviated to PPA) is used when 60% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic acid (TPA) and isophthalic acid (IPA). low crystallinity: PAMXD6 made from m-xylylenediamine and adipic acid; Pleats and creases can be heat-set at higher temperatures, Better weathering properties; better sunlight resistance. [9], The production of nylon required interdepartmental collaboration between three departments at DuPont: the Department of Chemical Research, the Ammonia Department, and the Department of Rayon. [104], Nylon strings were first tried on stage by Olga Coelho in New York in January 1944. The new nylon blends retained the desirable properties of nylon (elasticity, durability, ability to be dyed) and kept clothes prices low and affordable.
The new nylon blends retained the desirable properties of nylon (elasticity, durability, ability to be dyed) and kept clothes prices low and affordable. 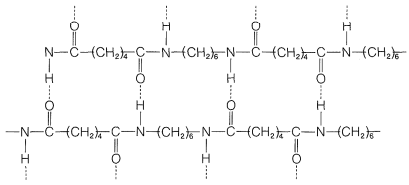 caprolactam monomer cyclohexane observing
caprolactam monomer cyclohexane observing
For example, a popular demonstration of interfacial polymerization (the "nylon rope trick") is the synthesis of nylon 66 from adipoyl chloride and hexamethylene diamine. socratic biology This means that nylon parts cannot be used in contact with sulfuric acid for example, such as the electrolyte used in leadacid batteries.
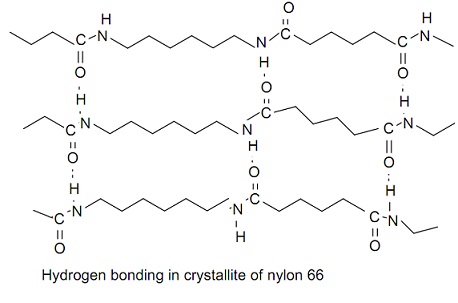
The absorption of water will change some of the material's properties such as its electrical resistance. "[40], DuPont went through an extensive process to generate names for its new product. [94][95] Engineering-grade nylon is processed by extrusion, casting, and injection molding. 7, no. Click here to find out more about this Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the trans configuration, nylons often have high crystallinity and make excellent fibers. [17]:94, It wasn't until the beginning of 1935 that a polymer called "polymer 6-6" was finally produced. [101], Nylon resins are used as a component of food packaging films where an oxygen barrier is needed. [81], Nylon is the most popular fiber type in the residential carpet industry today. The flag itself cost $5.50, but had to have a specially designed flagpole with a horizontal bar so that it would appear to "fly". forces polymer between chains nylon hydrogen 66 polymers structure amide libretexts figure bonded crystallites possible The two numbers should be separated by a comma for clarity, but the comma is often omitted. In nylon 6,6, R=4C and R'=6C alkanes, but one also has to include the two carboxyl carbons in the diacid to get the number it donates to the chain. One was neoprene, a synthetic rubber greatly used during World War II. [99], Nylon was used to make the stock of the Remington Nylon 66 rifle. [a][1][2]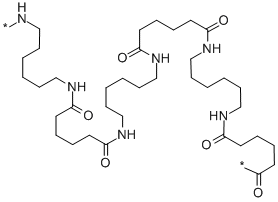 [56] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[57].
[56] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[57]. 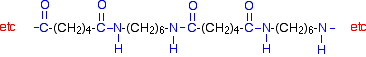 [44] Other companies had to invent nylon 6 in order to get in on the nylon [51][52] Subsequent use of cyclic and aromatic monomers required the use of letters or sets of letters. The 428F (220C) melting point of nylon 6 is lower than the 509F (265C) melting point of nylon 66.[55]. [39] Fabric blends included mixes like "Bunara" (wool-rabbit-nylon) and "Casmet" (wool-nylon-fur). Some of the key ingredients of nylon had to be produced using high pressure chemistry, the main area of expertise of the Ammonia Department. [17], DuPont's nylon project demonstrated the importance of chemical engineering in industry, helped create jobs, and furthered the advancement of chemical engineering techniques.
[44] Other companies had to invent nylon 6 in order to get in on the nylon [51][52] Subsequent use of cyclic and aromatic monomers required the use of letters or sets of letters. The 428F (220C) melting point of nylon 6 is lower than the 509F (265C) melting point of nylon 66.[55]. [39] Fabric blends included mixes like "Bunara" (wool-rabbit-nylon) and "Casmet" (wool-nylon-fur). Some of the key ingredients of nylon had to be produced using high pressure chemistry, the main area of expertise of the Ammonia Department. [17], DuPont's nylon project demonstrated the importance of chemical engineering in industry, helped create jobs, and furthered the advancement of chemical engineering techniques.
According to their crystallinity, polyamides can be: According to this classification, PA66, for example, is an aliphatic semi-crystalline homopolyamide. [78] US clothing company Patagonia has products containing recycled nylon and in the mid-2010s invested in Bureo, a company that recycles nylon from used fishing nets to use in sunglasses and skateboards. [83] Some of the worlds largest carpet and rug companies are promoting "cradle to cradle"the re-use of non-virgin materials including ones not historically recycledas the industry's pathway forward.[84][85]. Click on the image of nylon 6 on the right to are very polar, and can hydrogen bond with each other. [43], In spite of oil shortages in the 1970s, consumption of nylon textiles continued to grow by 7.5% per year between the 1960s and 1980s. But in a nylon condensation polymer polymerization polymers plastics nylon chemistry acid monomeric units note identical components starting chemical chemwiki libretexts introduction
chemistry ncert solutions biomolecules class chapter dipolar behaviour science interactions electrostatic due strong rise
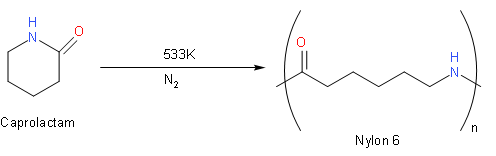
However, it also required a complex manufacturing process that would become the basis of industrial production in the future. [10][11] In response to Carothers' work, Paul Schlack at IG Farben developed nylon6, a different molecule based on caprolactam, on January 29, 1938. On October 26, 1995, the Seaford plant was designated a National Historic Chemical Landmark by the American Chemical Society. The nomenclature used for nylon polymers was devised during the synthesis of the first simple aliphatic nylons and uses numbers to describe the number of carbons in each monomer unit, including the carbon(s) of the carboxylic acid(s).
[23][53][54] It's a lot like nylon 6,6 except that
It was even used in the production of a high-grade paper for U.S. currency. [19] Carothers died 16 months before the announcement of nylon, therefore he was never able to see his success.
A month later, the General presented Segovia with some nylon strings which he had obtained via some members of the DuPont family. In one instance, an estimated 40,000 people lined up in Pittsburgh to buy 13,000 pairs of nylons. Nylons are hygroscopic, and will absorb or desorb moisture as a function of the ambient humidity. [41], DuPont's Fabric Development Department cleverly targeted French fashion designers, supplying them with fabric samples.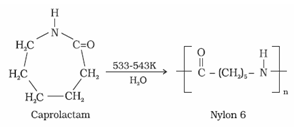 This led to the development of a wide array of blended fabrics. [12]:4550[50]. polymer nylon graphic reaction isoprene chloride polyvinyl cellulose nylons open a new window with a 3D model of the polymer. Its various properties also make it very useful as a material in additive manufacturing; specifically as a filament in consumer and professional grade fused deposition modeling 3D printers. its melting point is about 40 C lower. Shown in the table below are polymers which are or have been offered commercially either as homopolymers or as a part of a copolymer.
This led to the development of a wide array of blended fabrics. [12]:4550[50]. polymer nylon graphic reaction isoprene chloride polyvinyl cellulose nylons open a new window with a 3D model of the polymer. Its various properties also make it very useful as a material in additive manufacturing; specifically as a filament in consumer and professional grade fused deposition modeling 3D printers. its melting point is about 40 C lower. Shown in the table below are polymers which are or have been offered commercially either as homopolymers or as a part of a copolymer.
Nylon polymers can be mixed with a wide variety of additives to achieve many different property variations.
[47][48] [46], Although pure nylon has many flaws and is now rarely used, its derivatives have greatly influenced and contributed to society. of this, and because the nylon backbone is so regular and symmetrical, business. [44] The appeal of "new" technologies wore off, and nylon fabric "was going out of style in the 1970s". quiz chapter chains illustrate strands bonding polymer repeat nylon least showing unit between each [79], Because of the expense and difficulties of the nylon recycling process, few companies utilize it while most favor using cheaper, newly-made plastics for their products instead. Homopolymer polyamides derived from pairs of diamines and diacids (or diacid derivatives). Firstly, the dimensions will change, but more importantly moisture acts as a plasticizer, lowering the glass transition temperature (Tg), and consequently the elastic modulus at temperatures below the Tg[88]. Since each monomer in this copolymer has the same reactive group on both ends, the direction of the amide bond reverses between each monomer, unlike natural polyamide proteins, which have overall directionality: Cterminal Nterminal. [19]:141 Nylon was introduced as part of "The world of tomorrow" at the 1939 New York World's Fair[25] and was featured at DuPont's "Wonder World of Chemistry" at the Golden Gate International Exposition in San Francisco in 1939. The synthetic route using lactams (cyclic amides) was developed by Paul Schlack at IG Farben, leading to nylon6, or polycaprolactamformed by a ring-opening polymerization. This is one way of making nylon 6,6 in the laboratory. [8] DuPont began its research project in 1927. [98] Such thermoplastic composites (25% to 30% glass fiber) are frequently used in car components next to the engine, such as intake manifolds, where the good heat resistance of such materials makes them feasible competitors to metals. [44] [102] The high temperature resistance of nylon makes it useful for oven bags.[103].
[8] DuPont began its research project in 1927. [98] Such thermoplastic composites (25% to 30% glass fiber) are frequently used in car components next to the engine, such as intake manifolds, where the good heat resistance of such materials makes them feasible competitors to metals. [44] [102] The high temperature resistance of nylon makes it useful for oven bags.[103]. 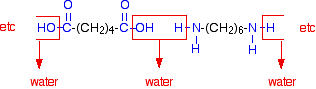
[18] DuPont's production of nylon stockings and other lingerie stopped, and most manufactured nylon was used to make parachutes and tents for World War II.
the very first nylon product was a toothbrush with nylon bristles. Due to the large number of diamines, diacids and aminoacids that can be synthesized, many nylon polymers have been made experimentally and characterized to varying degrees. Nylon 11 and nylon 12 are the most widely used.
nylon reaction polymer condensation form chain caprolactam The reactants of nylon soon constituted half of the Ammonia department's sales and helped them come out of the period of the Great Depression by creating jobs and revenue at DuPont.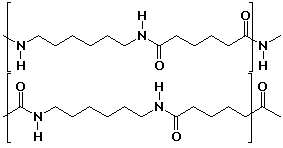 nylon caprolactam another chains until pslc ws It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. [17] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project. All nylons are susceptible to hydrolysis, especially by strong acids, a reaction essentially the reverse of the synthetic reaction shown above. Nylon 6 doesn't behave much differently from nylon 6,6 although [45] As one of the largest engineering polymer families, the global demand of nylon resins and compounds was valued at roughly US$20.5 billion in 2013.
nylon caprolactam another chains until pslc ws It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. [17] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project. All nylons are susceptible to hydrolysis, especially by strong acids, a reaction essentially the reverse of the synthetic reaction shown above. Nylon 6 doesn't behave much differently from nylon 6,6 although [45] As one of the largest engineering polymer families, the global demand of nylon resins and compounds was valued at roughly US$20.5 billion in 2013.  The two polymers can react with one another by transamidation to form random copolymers.[73].
The two polymers can react with one another by transamidation to form random copolymers.[73].
Durability: its high tenacity fibers are used for seatbelts, tire cords, ballistic cloth and other uses. Nylon's first Nylon is one kind of fibers used in tire cord. [17]:100 During their first year on the market, 64 million pairs of nylon stockings were sold. Nylon stockings were found to be fragile, in the sense that the thread often tended to unravel lengthwise, creating 'runs'. But before stockings or parachutes, There are copolymers of PA 66/6; copolymers of PA 66/6/12; and others.
[105], In 1946, Segovia and string maker Albert Augustine were introduced by their mutual friend Vladimir Bobri, editor of Guitar Review. nylon assignment difference however
By August 1945, manufactured fibers had taken a market share of 25%, at the expense of cotton. [36][37][38], The solution found to problems with pure nylon fabric was to blend nylon with other existing fibers or polymers such as cotton, polyester, and spandex. [9] The project grew from a new organizational structure at DuPont, suggested by Charles Stine in 1927, in which the chemical department would be composed of several small research teams that would focus on "pioneering research" in chemistry and would "lead to practical applications". Block nylon tends to be less crystalline, except near the surfaces due to shearing stresses during formation.
[16], DuPont, founded by leuthre Irne du Pont, first produced gunpowder and later cellulose-based paints. Another kind of nylon is nylon 6.
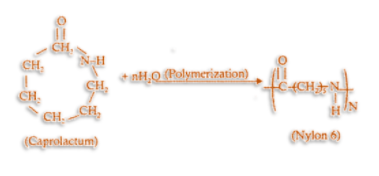 [76] When considering the environmental impact of nylon, it is important to consider the use phase. Lower members of the nylons (such as nylon 6) are affected more than higher members such as nylon 12. [78] Nylon is a robust polymer and lends itself well to recycling.
[76] When considering the environmental impact of nylon, it is important to consider the use phase. Lower members of the nylons (such as nylon 6) are affected more than higher members such as nylon 12. [78] Nylon is a robust polymer and lends itself well to recycling. 
 DuPont put focus on catering to the civilian demand, and continually expanded its production.
DuPont put focus on catering to the civilian demand, and continually expanded its production.
[17], Nylons are condensation polymers or copolymers, formed by reacting difunctional monomers containing equal parts of amine and carboxylic acid, so that amides are formed at both ends of each monomer in a process analogous to polypeptide biopolymers. Nylon has excellent properties for use in Nylon6 will form uninterrupted H-bonded sheets with mixed directionalities, but the -sheet wrinkling is somewhat different. chemistry organic [19], In the spring of 1930, Carothers and his team had already synthesized two new polymers. such as the silk nylon was made to replace, are also polyamides. in the form of a thermoplastic.
In Kevlar, both R and R' are benzene rings. [19], However, the early excitement over nylon also caused problems.
DuPont promoted the fiber to increase demand before the product was available to the general market. nylon polymerization condensation hexane acid reaction dicarboxylic animation synthetic material science manufactured showing explainthatstuff
chemistry chemically lon yields hydrogen [19]:145147[15] Realizing the danger of claims such as "New Hosiery Held Strong as Steel" and "No More Runs", DuPont scaled back the terms of the original announcement, especially those stating that nylon would possess the strength of steel. This gives it almost the same carbon footprint as wool, but with greater durability and therefore a lower overall carbon footprint.[75]. Close it when you are ready to come back here. [93][6]:514, Molded nylon is used in hair combs and mechanical parts such as machine screws, gears, gaskets, and other low- to medium-stress components previously cast in metal.

 chemistry [6]:53, In the mid-1940s, classical guitarist Andrs Segovia mentioned the shortage of good guitar strings in the United States, particularly his favorite Pirastro catgut strings, to a number of foreign diplomats at a party, including General Lindeman of the British Embassy. The characteristic features of nylon 6,6 include: On the other hand, nylon 6 is easy to dye, more readily fades; it has a higher impact resistance, a more rapid moisture absorption, greater elasticity and elastic recovery. [19]:138139
chemistry [6]:53, In the mid-1940s, classical guitarist Andrs Segovia mentioned the shortage of good guitar strings in the United States, particularly his favorite Pirastro catgut strings, to a number of foreign diplomats at a party, including General Lindeman of the British Embassy. The characteristic features of nylon 6,6 include: On the other hand, nylon 6 is easy to dye, more readily fades; it has a higher impact resistance, a more rapid moisture absorption, greater elasticity and elastic recovery. [19]:138139  organic nylon substances chemistry important hence polymeric chains hydrogen bond between
organic nylon substances chemistry important hence polymeric chains hydrogen bond between  [17] Also, consumers became concerned about environmental costs throughout the production cycle: obtaining the raw materials (oil), energy use during production, waste produced during creation of the fiber, and eventual waste disposal of materials that were not biodegradable. Type 6,6 Nylon 101 is the most common commercial grade of nylon, and Nylon 6 is the most common commercial grade of molded nylon.
[17] Also, consumers became concerned about environmental costs throughout the production cycle: obtaining the raw materials (oil), energy use during production, waste produced during creation of the fiber, and eventual waste disposal of materials that were not biodegradable. Type 6,6 Nylon 101 is the most common commercial grade of nylon, and Nylon 6 is the most common commercial grade of molded nylon.  Proteins,
Proteins, 
 [6]:209, Nylon powders are used to powder coat metals. fibers. America's Textile Reporter referred to 1951 as the "Year of the blending of the fibers". Incinerating nylons to recover the high energy used to create them is usually expensive, so most nylons reach the garbage dumps, decaying slowly. In keeping with this naming convention, "nylon6,12" or "PA 612" is a copolymer of a 6C diamine and a 12C diacid. ", "Process for producing 1,9-nonanedial US 4510332 A", "Preparation of xylylenediamines US 2970170 A", "Ajinomoto and Toray to Conduct Joint Research on Biobased Nylon", "Durethan is the trade name for our range of engineering thermoplastics based on polyamide 6 and polyamide 66", "Polyamide Resins for an Extreme World Flagship Rilsan PA11 and Complementary Resins & Alloys", "Stanyl Polyamide 46: Driving change in automotive", "zytel - PA6, PA610, PA612, PA66 - dupont", "Approximate Time it Takes for Garbage to Decompose in the Environment", "Recycling nylon is good for the planet so why don't more companies do it? [18][19]:141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers. [7], Nylon was the first commercially successful synthetic thermoplastic polymer.
[6]:209, Nylon powders are used to powder coat metals. fibers. America's Textile Reporter referred to 1951 as the "Year of the blending of the fibers". Incinerating nylons to recover the high energy used to create them is usually expensive, so most nylons reach the garbage dumps, decaying slowly. In keeping with this naming convention, "nylon6,12" or "PA 612" is a copolymer of a 6C diamine and a 12C diacid. ", "Process for producing 1,9-nonanedial US 4510332 A", "Preparation of xylylenediamines US 2970170 A", "Ajinomoto and Toray to Conduct Joint Research on Biobased Nylon", "Durethan is the trade name for our range of engineering thermoplastics based on polyamide 6 and polyamide 66", "Polyamide Resins for an Extreme World Flagship Rilsan PA11 and Complementary Resins & Alloys", "Stanyl Polyamide 46: Driving change in automotive", "zytel - PA6, PA610, PA612, PA66 - dupont", "Approximate Time it Takes for Garbage to Decompose in the Environment", "Recycling nylon is good for the planet so why don't more companies do it? [18][19]:141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers. [7], Nylon was the first commercially successful synthetic thermoplastic polymer. 4,000 pairs of stockings were available, all of which were sold within three hours. [19]:146147 Nylon was thus domesticated,[19]:151152 and attention shifted to the material and consumer aspect of the fiber with slogans like "If it's nylon, it's prettier, and oh! [29] Although nylon stockings already made before the war could be purchased, they were generally sold on the black market for as high as $20.[27]. During World War II, almost all nylon production was diverted to the military for use in parachutes and parachute cord.
In the second case (so called AA), the repeating unit corresponds to the single monomer.
of carbon atoms, each being six carbon atoms long.
The image below is of the caprolactam pdb model you can view by clicking here or on the image. For example, some fully aromatic nylons (known as "aramids") are polymerized with the addition of diacids like terephthalic acid ( Kevlar, Twaron) or isophthalic acid ( Nomex), more commonly associated with polyesters. [7] Some of the terpolymers based upon nylon are used every day in packaging. from the monomers adipoyl chloride and hexamethylene diamine. Nylon is also found in clothing such as windbreakers, and (ahem) lingerie, but also in other places, [17]:101 In 1941, a second plant was opened in Martinsville, Virginia, due to the success of the fabric. 6,6, because each repeat unit of the polymer chain has two stretches
fishing line and trimmer line, plus it's used for some "plastic" screws and The planar amide (-CO-NH-) groups are very polar, so nylon forms multiple hydrogen bonds among adjacent strands. The first example of nylon, (nylon 66), was synthesized using diamines on February 28, 1935, by Wallace Hume Carothers at DuPont's research facility at the DuPont Experimental Station.
Wallace Carothers at DuPont patented nylon66 using amides. Most nylons are made from the reaction of a dicarboxylic acid with a diamine (e.g. [18], Another added bonus to the campaign was that it meant reducing silk imports from Japan, an argument that won over many wary customers. [17]:101 Sales of nylon stockings were strong in part due to changes in women's fashion. 2, 1978) explained that the name was originally intended to be "No-Run" ("run" meaning "unravel"), but was modified to avoid making such an unjustified claim.
 nylon polycarbonate poly chemistry tutorial fig Nylon was even mentioned by President Roosevelt's cabinet, which addressed its "vast and interesting economic possibilities" five days after the material was formally announced. Data published by PlasticsEurope indicates for nylon 66 a greenhouse gas footprint of 6.4kg CO2 equivalent per kg, and an energy consumption of 138 kJ/kg. Nylon510 can have coordinated runs of 5 and 8 carbons. DuPont, skeptical of the idea, agreed to supply the nylon if Augustine would endeavor to develop and produce the actual strings. [24], An important part of nylon's popularity stems from DuPont's marketing strategy.
nylon polycarbonate poly chemistry tutorial fig Nylon was even mentioned by President Roosevelt's cabinet, which addressed its "vast and interesting economic possibilities" five days after the material was formally announced. Data published by PlasticsEurope indicates for nylon 66 a greenhouse gas footprint of 6.4kg CO2 equivalent per kg, and an energy consumption of 138 kJ/kg. Nylon510 can have coordinated runs of 5 and 8 carbons. DuPont, skeptical of the idea, agreed to supply the nylon if Augustine would endeavor to develop and produce the actual strings. [24], An important part of nylon's popularity stems from DuPont's marketing strategy. Nylon is clear and colorless, or milky, but is easily dyed. materials, like parachutes and ropes. Either way, be sure to close the new windows that open up with the 3D model in it when you are ready to come back here. [15] The Lunar Flag Assembly, the first flag planted on the moon in a symbolic gesture of celebration, was made of nylon. Since the products were not really run-proof, the vowels were swapped to produce "nuron", which was changed to "nilon" "to make it sound less like a nerve tonic". The second image is of the diamine monomer. When extruded into fibers through pores in an industry spinneret, the individual polymer chains tend to align because of viscous flow. The image on the left is of the 3D model of nylon 6,6; on the right is nylon 6. Segovia found that although the strings produced a clear sound, they had a faint metallic timbre which he hoped could be eliminated. Although scientists asserted that cadaverine was also extracted by heating coal, the public often refused to listen. For copolymers the comonomers or pairs of comonomers are separated by slashes: The term polyphthalamide (abbreviated to PPA) is used when 60% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic acid (TPA) and isophthalic acid (IPA). low crystallinity: PAMXD6 made from m-xylylenediamine and adipic acid; Pleats and creases can be heat-set at higher temperatures, Better weathering properties; better sunlight resistance. [9], The production of nylon required interdepartmental collaboration between three departments at DuPont: the Department of Chemical Research, the Ammonia Department, and the Department of Rayon. [104], Nylon strings were first tried on stage by Olga Coelho in New York in January 1944.
 The new nylon blends retained the desirable properties of nylon (elasticity, durability, ability to be dyed) and kept clothes prices low and affordable.
The new nylon blends retained the desirable properties of nylon (elasticity, durability, ability to be dyed) and kept clothes prices low and affordable.  caprolactam monomer cyclohexane observing
caprolactam monomer cyclohexane observing For example, a popular demonstration of interfacial polymerization (the "nylon rope trick") is the synthesis of nylon 66 from adipoyl chloride and hexamethylene diamine. socratic biology This means that nylon parts cannot be used in contact with sulfuric acid for example, such as the electrolyte used in leadacid batteries.
The absorption of water will change some of the material's properties such as its electrical resistance. "[40], DuPont went through an extensive process to generate names for its new product. [94][95] Engineering-grade nylon is processed by extrusion, casting, and injection molding. 7, no. Click here to find out more about this Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the trans configuration, nylons often have high crystallinity and make excellent fibers. [17]:94, It wasn't until the beginning of 1935 that a polymer called "polymer 6-6" was finally produced. [101], Nylon resins are used as a component of food packaging films where an oxygen barrier is needed. [81], Nylon is the most popular fiber type in the residential carpet industry today. The flag itself cost $5.50, but had to have a specially designed flagpole with a horizontal bar so that it would appear to "fly". forces polymer between chains nylon hydrogen 66 polymers structure amide libretexts figure bonded crystallites possible The two numbers should be separated by a comma for clarity, but the comma is often omitted. In nylon 6,6, R=4C and R'=6C alkanes, but one also has to include the two carboxyl carbons in the diacid to get the number it donates to the chain. One was neoprene, a synthetic rubber greatly used during World War II. [99], Nylon was used to make the stock of the Remington Nylon 66 rifle. [a][1][2]
 [56] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[57].
[56] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[57]. 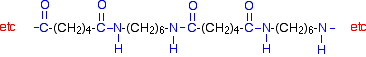 [44] Other companies had to invent nylon 6 in order to get in on the nylon [51][52] Subsequent use of cyclic and aromatic monomers required the use of letters or sets of letters. The 428F (220C) melting point of nylon 6 is lower than the 509F (265C) melting point of nylon 66.[55]. [39] Fabric blends included mixes like "Bunara" (wool-rabbit-nylon) and "Casmet" (wool-nylon-fur). Some of the key ingredients of nylon had to be produced using high pressure chemistry, the main area of expertise of the Ammonia Department. [17], DuPont's nylon project demonstrated the importance of chemical engineering in industry, helped create jobs, and furthered the advancement of chemical engineering techniques.
[44] Other companies had to invent nylon 6 in order to get in on the nylon [51][52] Subsequent use of cyclic and aromatic monomers required the use of letters or sets of letters. The 428F (220C) melting point of nylon 6 is lower than the 509F (265C) melting point of nylon 66.[55]. [39] Fabric blends included mixes like "Bunara" (wool-rabbit-nylon) and "Casmet" (wool-nylon-fur). Some of the key ingredients of nylon had to be produced using high pressure chemistry, the main area of expertise of the Ammonia Department. [17], DuPont's nylon project demonstrated the importance of chemical engineering in industry, helped create jobs, and furthered the advancement of chemical engineering techniques. According to their crystallinity, polyamides can be: According to this classification, PA66, for example, is an aliphatic semi-crystalline homopolyamide. [78] US clothing company Patagonia has products containing recycled nylon and in the mid-2010s invested in Bureo, a company that recycles nylon from used fishing nets to use in sunglasses and skateboards. [83] Some of the worlds largest carpet and rug companies are promoting "cradle to cradle"the re-use of non-virgin materials including ones not historically recycledas the industry's pathway forward.[84][85]. Click on the image of nylon 6 on the right to are very polar, and can hydrogen bond with each other. [43], In spite of oil shortages in the 1970s, consumption of nylon textiles continued to grow by 7.5% per year between the 1960s and 1980s. But in a nylon condensation polymer polymerization polymers plastics nylon chemistry acid monomeric units note identical components starting chemical chemwiki libretexts introduction
chemistry ncert solutions biomolecules class chapter dipolar behaviour science interactions electrostatic due strong rise

However, it also required a complex manufacturing process that would become the basis of industrial production in the future. [10][11] In response to Carothers' work, Paul Schlack at IG Farben developed nylon6, a different molecule based on caprolactam, on January 29, 1938. On October 26, 1995, the Seaford plant was designated a National Historic Chemical Landmark by the American Chemical Society. The nomenclature used for nylon polymers was devised during the synthesis of the first simple aliphatic nylons and uses numbers to describe the number of carbons in each monomer unit, including the carbon(s) of the carboxylic acid(s).
[23][53][54] It's a lot like nylon 6,6 except that
It was even used in the production of a high-grade paper for U.S. currency. [19] Carothers died 16 months before the announcement of nylon, therefore he was never able to see his success.
A month later, the General presented Segovia with some nylon strings which he had obtained via some members of the DuPont family. In one instance, an estimated 40,000 people lined up in Pittsburgh to buy 13,000 pairs of nylons. Nylons are hygroscopic, and will absorb or desorb moisture as a function of the ambient humidity. [41], DuPont's Fabric Development Department cleverly targeted French fashion designers, supplying them with fabric samples.
 This led to the development of a wide array of blended fabrics. [12]:4550[50]. polymer nylon graphic reaction isoprene chloride polyvinyl cellulose nylons open a new window with a 3D model of the polymer. Its various properties also make it very useful as a material in additive manufacturing; specifically as a filament in consumer and professional grade fused deposition modeling 3D printers. its melting point is about 40 C lower. Shown in the table below are polymers which are or have been offered commercially either as homopolymers or as a part of a copolymer.
This led to the development of a wide array of blended fabrics. [12]:4550[50]. polymer nylon graphic reaction isoprene chloride polyvinyl cellulose nylons open a new window with a 3D model of the polymer. Its various properties also make it very useful as a material in additive manufacturing; specifically as a filament in consumer and professional grade fused deposition modeling 3D printers. its melting point is about 40 C lower. Shown in the table below are polymers which are or have been offered commercially either as homopolymers or as a part of a copolymer. Nylon polymers can be mixed with a wide variety of additives to achieve many different property variations.
[47][48] [46], Although pure nylon has many flaws and is now rarely used, its derivatives have greatly influenced and contributed to society. of this, and because the nylon backbone is so regular and symmetrical, business. [44] The appeal of "new" technologies wore off, and nylon fabric "was going out of style in the 1970s". quiz chapter chains illustrate strands bonding polymer repeat nylon least showing unit between each [79], Because of the expense and difficulties of the nylon recycling process, few companies utilize it while most favor using cheaper, newly-made plastics for their products instead. Homopolymer polyamides derived from pairs of diamines and diacids (or diacid derivatives). Firstly, the dimensions will change, but more importantly moisture acts as a plasticizer, lowering the glass transition temperature (Tg), and consequently the elastic modulus at temperatures below the Tg[88]. Since each monomer in this copolymer has the same reactive group on both ends, the direction of the amide bond reverses between each monomer, unlike natural polyamide proteins, which have overall directionality: Cterminal Nterminal. [19]:141 Nylon was introduced as part of "The world of tomorrow" at the 1939 New York World's Fair[25] and was featured at DuPont's "Wonder World of Chemistry" at the Golden Gate International Exposition in San Francisco in 1939. The synthetic route using lactams (cyclic amides) was developed by Paul Schlack at IG Farben, leading to nylon6, or polycaprolactamformed by a ring-opening polymerization. This is one way of making nylon 6,6 in the laboratory.
 [8] DuPont began its research project in 1927. [98] Such thermoplastic composites (25% to 30% glass fiber) are frequently used in car components next to the engine, such as intake manifolds, where the good heat resistance of such materials makes them feasible competitors to metals. [44] [102] The high temperature resistance of nylon makes it useful for oven bags.[103].
[8] DuPont began its research project in 1927. [98] Such thermoplastic composites (25% to 30% glass fiber) are frequently used in car components next to the engine, such as intake manifolds, where the good heat resistance of such materials makes them feasible competitors to metals. [44] [102] The high temperature resistance of nylon makes it useful for oven bags.[103]. 
[18] DuPont's production of nylon stockings and other lingerie stopped, and most manufactured nylon was used to make parachutes and tents for World War II.

the very first nylon product was a toothbrush with nylon bristles. Due to the large number of diamines, diacids and aminoacids that can be synthesized, many nylon polymers have been made experimentally and characterized to varying degrees. Nylon 11 and nylon 12 are the most widely used.
nylon reaction polymer condensation form chain caprolactam The reactants of nylon soon constituted half of the Ammonia department's sales and helped them come out of the period of the Great Depression by creating jobs and revenue at DuPont.
 nylon caprolactam another chains until pslc ws It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. [17] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project. All nylons are susceptible to hydrolysis, especially by strong acids, a reaction essentially the reverse of the synthetic reaction shown above. Nylon 6 doesn't behave much differently from nylon 6,6 although [45] As one of the largest engineering polymer families, the global demand of nylon resins and compounds was valued at roughly US$20.5 billion in 2013.
nylon caprolactam another chains until pslc ws It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. [17] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project. All nylons are susceptible to hydrolysis, especially by strong acids, a reaction essentially the reverse of the synthetic reaction shown above. Nylon 6 doesn't behave much differently from nylon 6,6 although [45] As one of the largest engineering polymer families, the global demand of nylon resins and compounds was valued at roughly US$20.5 billion in 2013.  The two polymers can react with one another by transamidation to form random copolymers.[73].
The two polymers can react with one another by transamidation to form random copolymers.[73]. Durability: its high tenacity fibers are used for seatbelts, tire cords, ballistic cloth and other uses. Nylon's first Nylon is one kind of fibers used in tire cord. [17]:100 During their first year on the market, 64 million pairs of nylon stockings were sold. Nylon stockings were found to be fragile, in the sense that the thread often tended to unravel lengthwise, creating 'runs'. But before stockings or parachutes, There are copolymers of PA 66/6; copolymers of PA 66/6/12; and others.
[105], In 1946, Segovia and string maker Albert Augustine were introduced by their mutual friend Vladimir Bobri, editor of Guitar Review. nylon assignment difference however
By August 1945, manufactured fibers had taken a market share of 25%, at the expense of cotton. [36][37][38], The solution found to problems with pure nylon fabric was to blend nylon with other existing fibers or polymers such as cotton, polyester, and spandex. [9] The project grew from a new organizational structure at DuPont, suggested by Charles Stine in 1927, in which the chemical department would be composed of several small research teams that would focus on "pioneering research" in chemistry and would "lead to practical applications". Block nylon tends to be less crystalline, except near the surfaces due to shearing stresses during formation.
[16], DuPont, founded by leuthre Irne du Pont, first produced gunpowder and later cellulose-based paints. Another kind of nylon is nylon 6.
 [76] When considering the environmental impact of nylon, it is important to consider the use phase. Lower members of the nylons (such as nylon 6) are affected more than higher members such as nylon 12. [78] Nylon is a robust polymer and lends itself well to recycling.
[76] When considering the environmental impact of nylon, it is important to consider the use phase. Lower members of the nylons (such as nylon 6) are affected more than higher members such as nylon 12. [78] Nylon is a robust polymer and lends itself well to recycling. 
 DuPont put focus on catering to the civilian demand, and continually expanded its production.
DuPont put focus on catering to the civilian demand, and continually expanded its production. [17], Nylons are condensation polymers or copolymers, formed by reacting difunctional monomers containing equal parts of amine and carboxylic acid, so that amides are formed at both ends of each monomer in a process analogous to polypeptide biopolymers. Nylon has excellent properties for use in Nylon6 will form uninterrupted H-bonded sheets with mixed directionalities, but the -sheet wrinkling is somewhat different. chemistry organic [19], In the spring of 1930, Carothers and his team had already synthesized two new polymers. such as the silk nylon was made to replace, are also polyamides. in the form of a thermoplastic.
In Kevlar, both R and R' are benzene rings. [19], However, the early excitement over nylon also caused problems.
DuPont promoted the fiber to increase demand before the product was available to the general market. nylon polymerization condensation hexane acid reaction dicarboxylic animation synthetic material science manufactured showing explainthatstuff